Anti-CAR Linker Antibody - Efficient Universal CAR Detection Reagent
Published On 04/23/2025 2:25 PM

What is CAR-T Cell Therapy?
CAR-T cell therapy is as a type of immunotherapy that teaches T cells to recognize and destroy cancer. CAR-T is named after a mythical creature called the chimera and refers to Chimeric Antigen Receptor T-Cell, which is characterized by genetic modification to obtain a personalized therapeutic method for carrying T cells that recognize tumor antigens. CAR-T is able to sense cells with specific proteins on its surface through its own receptors, lock infected cells and cancer cells in this way, and then kill them. CAR-T cell therapy has been validated in patients with acute lymphoblastic leukemia, and personalized treatment produces significant results.
With a deeper understanding of the immune system, the application scope of CAR technology is continuously expanding, and the development of novel CAR cell therapies is becoming a hot topic. These include CAR-NK, CAR-M, CAR-Treg, and CAR-γδ. Compared to the potential adverse effects and limitations in treating solid tumors associated with CAR-T therapy, these new CAR cell therapies offer distinct advantages, infusing new vitality into the development of immunotherapies.
With a deeper understanding of the immune system, the application scope of CAR technology is continuously expanding, and the development of novel CAR cell therapies is becoming a hot topic. These include CAR-NK, CAR-M, CAR-Treg, and CAR-γδ. Compared to the potential adverse effects and limitations in treating solid tumors associated with CAR-T therapy, these new CAR cell therapies offer distinct advantages, infusing new vitality into the development of immunotherapies.
Basic Structure of CAR
Whether it is CAR-T, CAR-NK, or CAR-M, they all share a similar CAR structure. The CAR structure primarily consists of three parts: the extracellular domain, the transmembrane domain, and the intracellular domain.- Antigen-Binding Domain: This is typically a single-chain variable fragment (scFv), which is formed by connecting the variable heavy chain (VH) and variable light chain (VL) of an antibody with a linker. The scFv is responsible for recognizing tumor antigens. A hinge region connects the scFv to the transmembrane domain, providing flexibility to overcome spatial hindrance and allowing the scFv to access the target epitope.
- Transmembrane Domain: Its primary function is to ensure that the CAR is stably embedded in the cell membrane.
- Intracellular Domain: This includes co-stimulatory domains and signaling domains, which activate and enhance the immune cell response.
- Transmembrane Domain: Its primary function is to ensure that the CAR is stably embedded in the cell membrane.
- Intracellular Domain: This includes co-stimulatory domains and signaling domains, which activate and enhance the immune cell response.
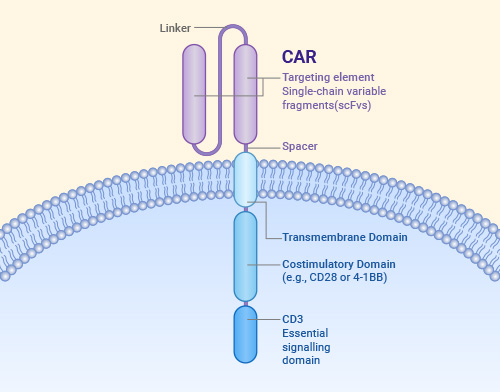
Rodríguez-Lobato LG, et al. Front Oncol. 2020.
Why Perform CAR Detection?
Currently, as more and more CAR-based cell therapies enter clinical research stages, the detection of CAR expression has become a critical aspect of drug quality control. This directly impacts treatment outcomes, and relevant regulations have set clear requirements for CAR positivity testing.Therefore, to ensure strict control over all stages—from early research phases involving CAR cell preparation and functional testing, through CMC phases for cell quality and purity assessment, to clinical testing phases for in vivo cell monitoring and PK (Pharmacokinetics) studies—it is crucial to develop accurate, simple, and universal CAR positivity detection reagents.
Common CAR detection methods include anti-human (or mouse) IgG F(ab')2 polyclonal antibodies, anti-idiotypic antibodies, and Protein L. While each method has its advantages, they also have some limitations. For example, they often require the development of specific antibodies for each CAR, which can be costly, and they can only be used to detect known antigens*.
Comparison of Existing CAR Detection Methods
| Detection Method | Detection Mechanism | Advantages | Limitations |
|---|---|---|---|
| Anti-human F(ab')2 pAb and Anti-mouse F(ab')2 pAb | Binds to IgG F(ab')2 fragments |
|
|
| Anti-idiotypic antibodies | Specifically binds to the antigen-binding region of scFv |
|
|
| rh-protein | Specifically binds to the antigen-binding region of scFv |
|
|
| Protein L | Binds to antibody kappa light chains |
|
|
| > Our Products Linker-specific mAbs |
Detect CAR linker |
|
|
Linker-Specific Monoclonal Antibodies from CUSABIO, Available for Purchase at Biohippo
Since most CAR constructs contain single-chain variable fragments (scFvs) with either Whitlow or G4S linkers, the Whitlow linker (GSTSGSGKPGSGEGSTKG) is primarily used in CAR constructs targeting hematological malignancies, such as CD19 CAR [1], while the G4S linker (glycine4-serine) is commonly used in CARs targeting solid tumor antigens, such as HER2 and CEA [2]. Developing specific monoclonal antibodies against these two linkers allows for the detection of a wide range of CARs.Based on this technical background, CUSABIO now introduces a new set of highly efficient and broadly applicable CAR positivity detection tools—Anti-Whitlow Linker Antibody and Anti-G4S Linker Antibody.
| Product Name | Cat No. | Clone Number |
|---|---|---|
| Whitlow linker Monoclonal Antibody | BHA10564641 | 18A3G5 |
| Whitlow linker Monoclonal Antibody, FITC conjugated | BHA10564645 | |
| G4S linker Monoclonal Antibody | BHA10564642 | 11A12E7 |
| G4S linker Monoclonal Antibody | BHA10564643 | 12F11C10 |
| G4S linker Monoclonal Antibody | BHA10564644 | 14A10E7 |
| G4S linker Monoclonal Antibody, FITC conjugated | BHA10564646 |
Product Features

Product Data
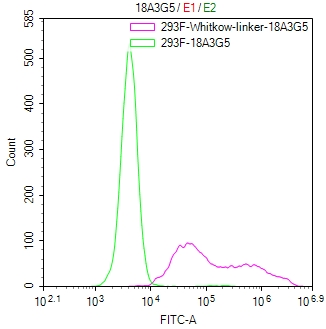
Figure 1. The Binding Activity of Whitlow linker with Anti-Whitlow linker antibody. Untransfected HEK-293F cells (green line) and transfected scFv-based Anti-CD19 CAR containing a Whitlow/218 linker stable cells (red line) were stained with anti-Whitlow linker antibody (2µg/1*10^6), washed and then followed by FITC-conjugated anti-Mouse IgG Fc antibody and analyzed with flow cytometry.
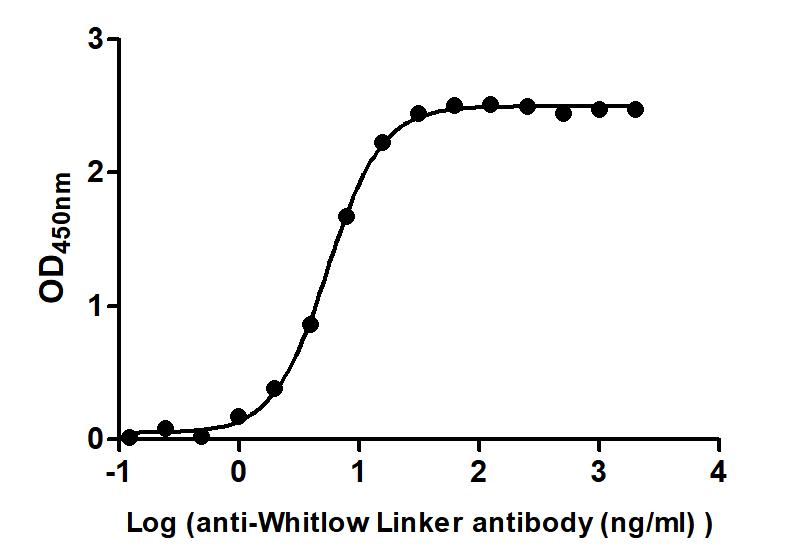
Figure 2. The Binding Activity of Whitlow linker with Anti-Whitlow linker antibody. Immobilized scFv-based Anti-CD19 CAR recombinant antibody at 2 μg/mL can bind Anti-Whitlow linker antibody. The EC50 is 5.147 to 5.761 ng/mL.

Figure 3. The Binding Activity of G4S linker with Anti-G4S linker antibody. Untransfected HEK-293F cells (green line) and transfected scFv-based Anti-CD19 CAR containing a G4S linker stable cells (red line) were stained with anti-G4S linker antibody (2µg/1*106), washed and then followed by APC-conjugated anti-Mouse IgG Fc antibody and analyzed with flow cytometry.
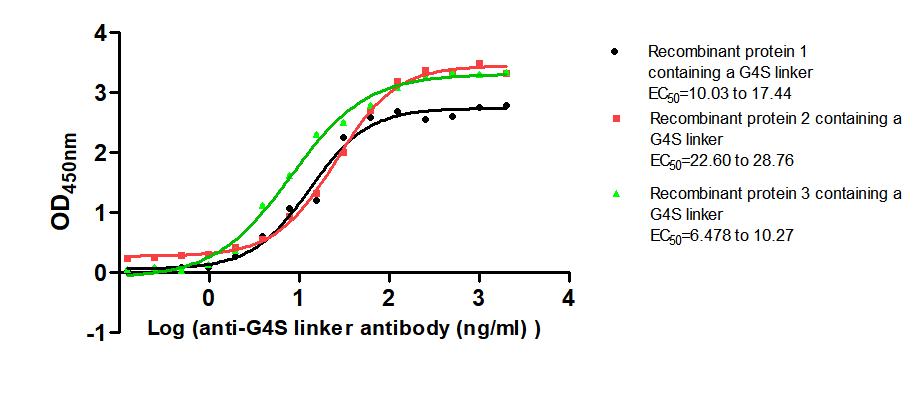
Figure 4. The Binding Activity of G4S linker with Anti-G4S linker antibody. Three immobilized recombinant proteins containing G4S linker at 2 μg/mL can bind Anti-G4S linker antibody. The EC50s are 10.03 to 17.44 ng/mL, 22.60 to 28.76 ng/mL, and 6.478 to 10.27 ng/mL, respectively.
References
[1] B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood. 2012.
[2] CAR-T cell potency: from structural elements to vector backbone components. Biomark Res. 2022.
This entry was posted in
Product Literature
,Application and Technique Notes
 Loading ....
Loading ....
