What are the key benefits of using CellTrypase?CellTrypase is a superior quality, GMP-grade recombinant trypsin-like enzyme for gentle and efficient cell dissociation designed for biopharmaceutical manufacturing.
• Animal Origin-Free & GMP-Compliant: Manufactured without animal-derived components or antibiotics. Available in both GMP-grade and ISO 9001 R&D-grade formats.
• Gentle Cell Dissociation: Maintains high cell viability and functionality across a wide range of cell types
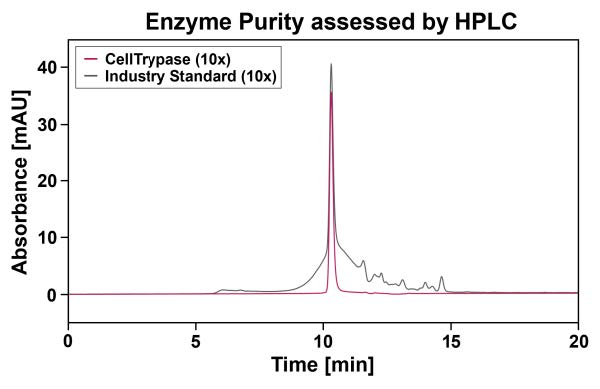
• High Purity & Consistency: ≥95% purity specified. Confirmed by HPLC. The sharp, well-defined chromatographic peak indicates minimal degradation products or side activities.
• Direct Replacement: Compatible with existing trypsin-based protocols. Enables 1:1 substitution without process revalidation.
How can I get a free sample to test CellTrypase in my process?
c-LEcta, a Kerry Company, offers 100 mL samples for process development, testing, and qualification. Please reach out to your eBiohippo contact or request your free sample directly via orders@biohippo.com
What can CellTrypase be used for?CellTrypase is suitable for a wide range of applications in biopharma manufacturing and research, including:
- Passaging and expansion of adherent cell lines (e.g. Vero, MDCK, HEK293,…)
- Cell dissociation in vaccine and viral vector production for cell and gene therapies
- Processing of stem cells (e.g. iPSCs, hESCs and organoids)
- Isolation of primary cells (e.g. fibroblasts, keratinocytes, chondrocytes, neuronal cells)
- Cell banking and tissue engineering workflows
- General life science R&D use
Is CellTrypase suitable for GMP Manufacturing?
Yes. CellTrypase is available in a GMP-grade specifically developed for use in clinical and commercial biomanufacturing processes.
CellTrypase GMP-grade is manufactured and distributed under a certified Quality Management System in compliance with Good Manufacturing and Distribution Practice (GMP/GDP) according to the EXCiPACT® Certification Standard for pharmaceutical excipients. A Drug Master File (DMF) submission is part of our regulatory roadmap to support future global filings
Is CellTrypase an alternative to TrypLE™*?Yes. CellTrypase is intended as a direct alternative to TrypLE™ and other trypsin-based cell dissociation reagents. It offers equivalent performance, is compatible with existing protocols, and is available in GMP- and R&D-grades that match TrypLE™ Select as well as CTS and TrypLE™ Express, respectively.*
Produced entirely in-house by c-LEcta, CellTrypase ensures high purity and secure supply.
* TrypLE™ is a registered trademark owned by Thermo Fisher Scientific Inc.
Can CellTrypase replace porcine trypsin? Yes, CellTrypase is an ideal alternative to porcine trypsin. Produced under controlled, consistent conditions and formulated as a highly purified fungal enzyme, CellTrypase is inherently less reactive and so more gentle on cells. It offers:
- Comparable enzymatic activity
- Superior purity (≥95% by HPLC)
- Gentler cell dissociation with lower toxicity
- No need for inactivation
- Compatibility with existing protocols
Are there different quality grades for CellTrypase?Yes. CellTrypase is available in two quality grades tailored to different use cases:
GMP-grade: For clinical-stage development and commercial manufacturing. Produced in compliance with the quality management system according to GMP standards.
R&D-grade: For early-stage development, process optimization, and non-clinical applications. Manufactured under ISO 9001 standard.
Both grades are offered as
- 100 mL and 500 mL bottles
- 1x ready-to-use solutions or 10x concentrated stock formats
All SKUs are manufactured using the same production process and meet identical specifications, ensuring consistent quality and performance across the entire portfolio.
Is CellTrypase animal component-free?Yes. CellTrypase is entirely free from animal-derived components. The recombinant enzyme is manufactured by microbial fermentation and produced on animal origin-free equipment.
All raw materials, equipment, and packaging are certified as animal origin- and TSE/BSE-free. This ensures full compliance with regulatory expectations and minimizes the risk of contamination.
What sets CellTrypase apart from other recombinant trypsin-like enzymes on the market?A proprietary GMP-compliant manufacturing process that meets the needs of customers looking for the highest quality and supply chain standards for their raw materials.
In-house production at our GMP-certified facility in Germany ensures full traceability and adherence to GMP/GDP requirements and offers a high security of supply. Gentle manufacturing steps preserve product integrity throughout the entire production process. The result is a high-quality enzyme solution. A purity of ≥95% is specified as a release-relevant parameter, ensuring consistent performance in sensitive cell culture applications
CellTrypase is also animal origin-free from start to finish: Produced on equipment in facilities where the use of animal-derived materials is strictly prohibited.
How is security and traceability for CellTrypase ensured?
CellTrypase is manufactured under full control by c-LEcta in collaboration with qualified partners – from microbial fermentation to warehousing and distribution of the final product. This setup ensures:
- End-to-end traceability across all production stages
- Full transparency for audits and regulatory submissions
- Consistent quality and documentation
- Reliable global availability through secure and scalable supply chains
- With no dependency on external bulk suppliers, c-LEcta guarantees consistent quality, regulatory confidence, and long-term supply security as well as traceability.
How is CellTrypase handled and shipped to ensure product integrity?CellTrypase is filled into PET containers with HDPE lids and secured with a tamper-evident shrink seal. The primary packaging material complies with USP Class VI and 21 CFR standards for pharmaceutical use.
The product's high stability allows shipment at ambient temperature.
What does EXCiPACT®-compliant GMP mean?
EXCiPACT® GMP is an internationally recognized quality certification for pharmaceutical excipients , but its principles are equally relevant for processing aids used in pharmaceutical manufacturing. The quality management system ensures that products, such as CellTrypase, are produced, stored, and distributed under controlled, traceable, and auditable conditions which meet the regulatory requirements of the pharmaceutical industry.
To achieve EXCiPACT® GMP certification, a company must first implement and operate a certified ISO 9001 quality management system. EXCiPACT® then adds specific GMP and GDP requirements tailored for materials going into pharmaceutical processes. These include documentation, hygiene, equipment, training, storage, and shipping.
- Ensures pharma-grade quality
- Accepted by global regulatory authorities (EMA, FDA, ANSM)
- Includes GDP-compliant storage and shipping
- Ideal for processing aids like CellTrypase used in vaccine, CGT, and biologics manufacturing

 Loading ....
Loading ....