Cellular therapies for cancer have significantly impacted the field of immunotherapy. Among these, chimeric antigen receptor (CAR) engineering stands out. Initially applied to T cells in the 1990s, CARs combine T cells’ anticancer properties with antigen specificity, exemplifying personalized medicine. CAR-T cells have shown promise in treating hematological B-cell malignancies (Eshhar et al., 1993). However, CAR-T therapy faces limitations, including time-consuming production, complex logistics related to generating personalized autologous products for each patient, high costs, and a notable toxicity profile. While allogeneic CAR-T cells have been employed to mitigate some of these challenges, they introduce their own issues, including the risk of graft versus host disease (GVHD) even with human leukocyte antigen (HLA) matching. Additionally, limiting side effects involve a substantial risk of cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS). Given these challenges, researchers are exploring alternative immune effector cell populations as potential carriers for CARs. In recent years, natural killer cells have emerged as promising alternatives to modified T cells.
These NK cells were first discovered in mice in 1975 and were shown to selectively kill leukemia cells. Unlike T cells, NK cells operate through innate receptors, allowing them to eliminate cancerous cells without prior sensitization. Their function and activity are regulated solely by the balance of signals received from activating and inhibitory receptors (Rezvani et al., 2017) (See Fig.1). NK cells mediate target cell lysis through several distinct mechanisms, including cytotoxic granule exocytosis, upregulation of death ligand [removed]such as Fas and TRAIL), and production of cytokines like interferon gamma (IFN-γ). Furthermore, NK cells can also participate in antibody-dependent cell-mediated cytotoxicity (ADCC). In ADCC, monoclonal antibodies that target antigens expressed by tumor cells recruit NK cells through FcγR-Fc interactions, leading to NK cell activation and target cell lysis.

Fig.1. Natural Killer (NK) cells' immune response to cancer cells, involving a delicate balance of activating and inhibitory signals. Source of image: BPS Bioscience
Advantages of CAR-NK Cells:
-
Preserved Cytotoxicity: The addition of a CAR to NK cells redirects them toward antigen-specific targets, while their innate cytotoxicity remains intact even if target antigens change.
-
Minimal Side-Effects: Unlike CAR-T cells, which can cause severe toxicities, NK cells have not shown similar adverse effects.
-
Off-the-Shelf Production: CAR-NK cells can be generated from multiple sources, allowing for large-scale production and making “off-the-shelf” treatment a possibility.
-
No HLA Compatibility Requirement: Unlike CAR-T cells, which need HLA matching, CAR-NK cells can be used without this constraint.
CAR-NK Cell Production:
Commonly used sources for creating CAR-NK cells include NK-92 cell line, adult peripheral blood (AB) (Cat #: BHC18200327), umbilical cord blood (CB), and induced pluripotent stem cells (iPSCs) (Cat #: BHC14300002). While using autologous NK cells for CAR-NK production is feasible, their effectiveness against cancer might be restricted, and incorporating CAR engineering could pose challenges. Harvested NK cells, depending on their source, exhibit differences in maturation stages, viability, and anti-tumor capabilities. The pros and cons of each NK cell source are outlined as follows:
| NK Cell Source |
Pros |
Cons |
| NK-92 cell line |
- Highly cytotoxic
- Low inhibitory receptor expression
- Easy and quick in vitro expansion
|
- Limited proliferation & persistence in vivo
- Cannot trigger antibody-dependent cell-mediated cytotoxicity (ADCC)
- Small risk of tumor engraftment and therefore irradiation prior to infusion is a safety requirement
|
| Primary NK cells from Adult Peripheral Blood |
- More mature and possess great cytotoxic potential.
- Extended persistence in vivo |
- Labor intensive isolation and expansion in vitro
- Low yield of NK cells |
| Primary NK cells from Umbilical Cord Blood |
- High yield of NK cells
- Greater potential for in vitro expansion
- Extended persistence in vivo |
- Labor intensive isolation and expansion in vitro
- Immature phenotype and therefore inferior cytotoxic capability |
| iPSC |
- Easy and quick in vitro production of homogeneous NK cells
- Can more readily transduce with transgenes. |
- Immature phenotype and inferior cytotoxic capability |
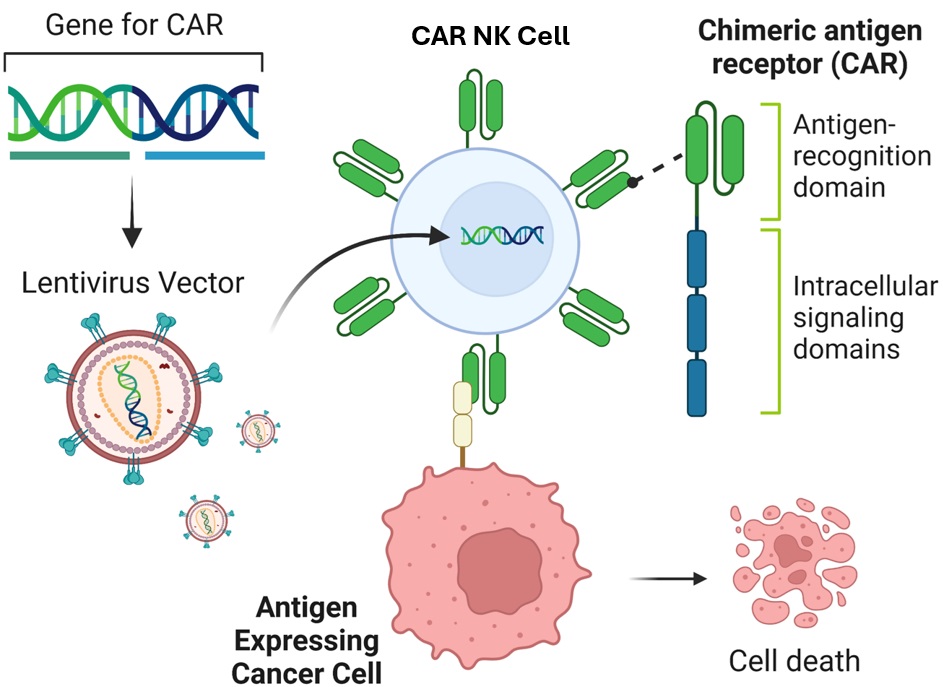
CAR constructs are comprised of three main components: an ectodomain for antigen recognition, a transmembrane linker, and an intracellular signaling domain. The ectodomain typically contains a single-chain variable fragment (scFv) that targets specific tumor cell antigens. When a CAR-expressing NK cell binds to the target antigen, the intracellular signaling domain triggers activation signals, leading to cytokine secretion and target cell lysis. CARs have evolved from first-generation constructs with CD3ζ signaling alone to third-generation constructs with multiple costimulatory domains (Sadelain, 2013). Fourth-generation CARs incorporate additional transgenic products for enhanced cytotoxicity and cytokine release. CAR-NK cells are generated through viral transduction, with lentiviral vectors being commonly used due to their ability to infect both dividing and non-dividing cells. CAR-NK cells demonstrate faster target cell conjugation and enhanced cytotoxicity compared to unmodified NK cells. Promising CAR targets include antigens like CD38, CD20, mesothelin, CD22, CD19, GD2, and HER2 (MacKay, 2020), with a focus on minimizing off-target effects for maximum clinical benefit.
Pre-clinical Data on CAR-NK Cell Therapy
Cytokine induced memory like (CIML) NK cells have been developed by activating primary NK cells from adult peripheral blood with IL-12, IL-15, and IL-18. These cells exhibit potent antitumor responses and have been utilized in the treatment of leukemia, inducing complete remission in these patients (Romee et al., 2016). Recently, these cells have been transduced with CAR constructs targeted against CD19 and have effectively controlled lymphoma burden in vivo (Gang et al., 2018). These memory like CAR-NK cells exhibited increased degranulation and specific killing compared to conventional CAR-NK cells.
Preclinical studies on CAR-NK cells have shown promising results. While primary NK cells are effective for CAR modification, most preclinical work has utilized the NK-92 cell line. Second and third generation CAR-NK cells from NK-92 cells have shown effectiveness in targeting different antigens and cancer cell types. Clinical trials for CAR-NK therapy are relatively recent, with only three published trials so far. These trials have demonstrated the safety and efficacy of CAR-NK cells in patients with multiple myeloma, acute myeloid leukemia, and CD19+ lymphoid tumors (Jiang et al., 2014; Tang et al., 2018; Liu et al., 2020).
Additionally, pilot clinical studies have shown notable responses, indicating the potential of CAR-NK therapy for various malignancies. There are currently 32 CAR-NK-based clinical trials registered in NIH.gov, with a majority ongoing in China, highlighting the growing interest and potential of CAR-NK cell-based therapeutics for cancer treatment (Moscarelli et al., 2022).
Challenges and Future Directions
CAR-NK cell therapy holds promise for cancer treatment, but several challenges need addressing for its clinical application. Firstly, current CAR constructs designed for CAR-T cells may not be optimal for NK cells due to differences in antigen binding and activation. Customized CAR constructs tailored for NK cell functions could enhance efficacy. Another hurdle is NK cell sensitivity to freeze-thaw processes, impacting their survival and cytotoxicity. Developing ideal cryopreservation and recovery protocols is crucial for "off-the-shelf" CAR-NK therapy. Moreover, CAR-NK cells lack persistence in vivo without cytokine support, necessitating strategies to enhance viability and prolong persistence, with IL-15 being preferred due to its favorable toxicity profile. Engineering CAR-NK cells for proper homing and trafficking to solid tumors is essential for effective treatment. Techniques like modifying chemokine receptors or incorporating cytokine genes aim to improve tumor infiltration. Additionally, CAR-NK cells face challenges in overcoming tumor resistance mechanisms, including inhibition by the tumor microenvironment (TME) and tumor cell-based resistance. Strategies such as knocking out inhibitory signaling genes or rendering CAR-NK cells resistant to TGF-β immunosuppression show promise in enhancing efficacy against resistant tumors. Addressing these challenges through innovative engineering approaches is vital for advancing CAR-NK cell therapy for cancer treatment.
Biohippo’s Solutions for Priming NK Cell Research
- Enriched and expanded NK cells from human PBMCs
- Ideal for ADCC or NK cell cytotoxicity assays
- Can be further expanded
- Two to three-fold exponential (x102 – 103) expansion of NK cells
- Purified NK cell molecules and ligands for in vitro ligand binding assays
- Multiple labels and tags available
- Reporter cells for co-culture assays to screen for inhibitors or ADCC modulators
- NK cell lines
- Biochemical and cell-based assay kits to screen for checkpoint inhibitors.
- For expression of CARs, NK molecules or ligands
- CRISPR/Cas9 lentiviruses for knocking out NK-related molecules.
- Neutralizing or agonistic antibodies targeting NK proteins and ligands.
- Bispecific FcGR3A-EGFR Antibody
- Cytokines suitable for cell culture applications including IL-2, IL-8, IL-15, IL-18, IL-21 and more.
References:
1. Eshhar Z, Waks T, Gross G, Schindler DG. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proceedings of the National Academy of Sciences. 1993;90(2):720–724. doi: 10.1073/pnas.90.2.720
2. Gang M, Marin ND, Wong P, et al. CAR-modified memory-like NK cells exhibit potent responses to NK-resistant lymphomas. Blood. 2020;136(20):2308. doi: 10.1182/BLOOD.2020006619
3. Jiang H, Zhang W, Shang P, et al. Transfection of chimeric anti-CD138 gene enhances natural killer cell activation and killing of multiple myeloma cells. Molecular Oncology. 2014;8(2):297–310. doi: 10.1016/j.molonc.2013.12.001
4. Liu E, Marin D, Banerjee P, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. New England Journal of Medicine. 2020;382(6):545–553. doi: 10.1056/NEJMoa1910607
5. MacKay M, Afshinnekoo E, Rub J, et al. The therapeutic landscape for cells engineered with chimeric antigen receptors. Nature Biotechnology. 2020;38(2):233–244. doi: 10.1038/s41587-019-0329-2
6. Moscarelli J, Zahavi D, Maynard R, Weiner LM. The Next Generation of Cellular Immunotherapy: Chimeric Antigen Receptor-Natural Killer Cells. Transplant Cell Ther. 2022 Oct;28(10):650-656. doi: 10.1016/j.jtct.2022.06.025. Epub 2022 Jul 3. PMID: 35788086; PMCID: PMC9547868.
7. Rezvani K, Rouce R, Liu E, Shpall E. Engineering Natural Killer Cells for Cancer Immunotherapy. Molecular Therapy. 2017;25(8):1769–1781. doi: 10.1016/j.ymthe.2017.06.012
8. Romee R, Rosario M, Berrien-Elliott MM, et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med. 2016;8(357):357ra123. doi: 10.1126/SCITRANSLMED.AAF2341
9. Sadelain M, Brentjens R, Rivière I. The basic principles of chimeric antigen receptor design. Cancer Discovery. 2013;3(4):388–398. doi: 10.1158/2159-8290.CD-12-0548
10.Tang X, Yang L, Li Z, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–1089.



 Loading ....
Loading ....
